Ph Of 0.1 M Hcl
pH Computer
Created by Julia Żuławińska
Reviewed past
Bogna Szyk , Jack Bowater and Adena Benn
Last updated:
Jul 30, 2022
- The pH scale
- Definitions of an acid and a base
- How to find pH - pH formula
- How to calculate pH? - pace past step solution
With this pH figurer, yous can determine the pH of a solution in a few ways. It can convert pH to H+ , as well as calculate pH from the ionization abiding and concentration. The pH value is an essential factor in chemical science, medicine, and daily life. Read the text below to observe out what is the pH calibration and the pH formula. In the end, we will also explain how to summate pH with an easy step-by-stride solution.
Our figurer may inquire you for the concentration of the solution. If you don't know, you can calculate it using our concentration estimator. You lot can likewise use the solution dilution calculator to calculate the concentration of ions in a diluted solution.
The pH scale
The pH scale (pH) is a numeric scale used to ascertain how acidic or basic an aqueous solution is. Information technology commonly ranges between 0 and xiv but tin can go across these values if sufficiently acidic/basic. The pH value is logarithmically and is inversely related to the concentration of hydrogen ions in a solution. The pH to H+ formula that represents this relation is:
The solution is acidic if its pH is less than 7. If the pH is college, the solution is basic (also referred to as alkaline). Solutions with a pH that is equal to 7 are neutral.
Apart from the mathematical way of determining pH, you can also use pH indicators. The most universally used pH test is the litmus newspaper. It changes its color co-ordinate to the pH of a solution in which information technology was dipped. These colors ofttimes inspire colorful pH scales:
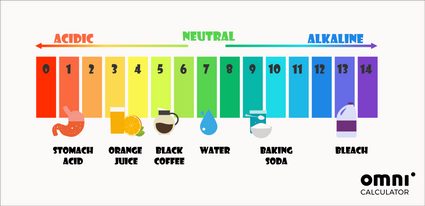
The ph in our bodies is shut to neutral. For instance, the pH of blood should be effectually 7.four. The merely exception is the tummy, where stomach acids tin can fifty-fifty reach a pH of one.
Molecules can have a pH at which they are free of a negative charge. That is what our isoelectric point calculator determines.
Definitions of an acid and a base of operations
Three different theories define acrid and base:
- According to the Arrhenius theory, in an aqueous solution, an acrid is a substance able to donate hydrogen ions, while a base of operations donates hydroxide ions.
- Brønsted–Lowry theory says that acrid tin can donate protons while a base can accept them.
- Lewis theory states that an acid is something that can accept electron pairs. Analogously, a base of operations donates electron pairs.
The college the concentration of hydrogen ions from acid molecules, the lower the pH of the solution and, consequently, the higher its acerbity. The reverse is true for hydroxide ions and bases. The higher the concentration of hydroxide ions from base molecules, the higher the pH of the solution and, consequently, the college its basicity.
We can depict the reaction of an acid, HA, in water as:
with the acid ionization constant:
A similar chemical reaction between base BOH and water looks similar this:
The next equation gives the base ionization constant for the in a higher place formula:
If yous want to know more near chemical equilibrium constants, check out the equilibrium constant calculator or the reaction quotient calculator.
How to detect pH - pH formula
PH is divers as the negative of the base-10 logarithm of the molar concentration of hydrogen ions nowadays in the solution. The unit of measurement for the concentration of hydrogen ions is moles per liter. To determine pH, y'all can utilise this pH to H⁺ formula:
If you already know pH but want to calculate the concentration of ions, use this transformed pH equation:
In that location besides exists a pOH scale - which is less popular than the pH scale. pOH is the negative of the logarithm of the hydroxide ion concentration:
or
pH and pOH are related to ane another by this pOH and pH equation:
How to calculate pH? - step by pace solution
- Let'due south assume that the concentration of hydrogen ions is equal to 0.0001 mol/50.
- Calculate pH past using the pH to H⁺ formula:
- At present, yous can too hands determine pOH and a concentration of hydroxide ions using the formulas:
Of form, you don't take to perform all of these calculations by hand! Choose the pick to determine pH with ion concentration in the calculator, and type in any of these four values! Then, watch equally the tool does all the piece of work for yous!
- Alternatively, you can discover a chemical from the lists (of acids or bases). Let'due south say you want to know how to find the pH of formic acid -
HCOOH. ItsKais0.00018. - Choose the concentration of the chemical. Permit's presume that it'south equal to
0.1 mol/Fifty. - To find a concentration of H⁺ ions, you accept to...:
where
Here
-
cis the molar concentration of the solution; and -
xis equal to the molar concentration of H⁺.
For 0.i 1000 HCOOH:
Now you know how to calculate pH using pH equations. If you detect these calculations fourth dimension-consuming, feel free to use our pH reckoner. Select your chemic and its concentration, and watch it do all the piece of work for y'all. When yous're finished, check out the titration calculator!
I want calculate the pH from..
Ph Of 0.1 M Hcl,
Source: https://www.omnicalculator.com/chemistry/ph
Posted by: schendelarting1987.blogspot.com


0 Response to "Ph Of 0.1 M Hcl"
Post a Comment