Sih4 Lewis Structure Molecular Geometry
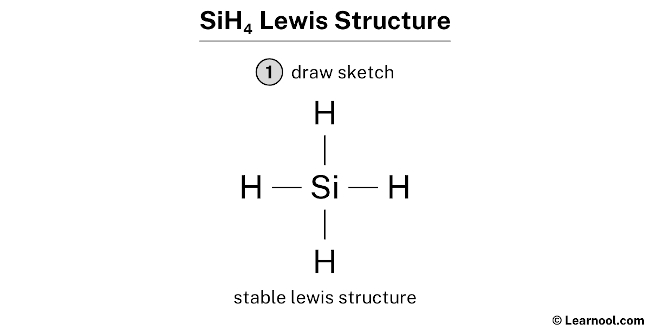
SiH4 (silane) has one silicon atom and 4 hydrogen atoms.
In the lewis structure of SiH4, there are four single bonds effectually the silicon atom, with four hydrogen atoms attached to it, and none of the atoms has a alone pair.
Steps
Hither's how you can describe the SiHfour lewis structure step by pace.
Step #ane: draw sketch
Step #2: mark lone pairs (if there are)
Permit'due south pause down each step in item.
#1 Depict Sketch
- First, make up one's mind the total number of valence electrons
In the periodic table, silicon lies in grouping 14, and hydrogen lies in group one.
Hence, silicon has four valence electrons and hydrogen has i valence electron.
Since SiH4 has one silicon atom and four hydrogen atoms, so…
Valence electrons of one silicon cantlet = 4 × 1 = 4
Valence electrons of 4 hydrogen atoms = ane × 4 = 4
And the total valence electrons = 4 + four = eight
Learn how to detect: Silicon Valence Electrons and Hydrogen Valence Electrons
- 2d, find the full electron pairs
We have a total of 8 valence electrons. And when we split up this value by ii, we get the value of total electron pairs.
Full electron pairs = total valence electrons ÷ 2
So the total electron pairs = 8 ÷ two = four
- Tertiary, decide the primal atom
Here hydrogen can not be the central atom. Because the key atom is bonded with at to the lowest degree two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than than one bond.
Hence, here we have to assume that the central atom is silicon.
Therefore, place silicon in the center and hydrogens on either side.
- And finally, depict the rough sketch
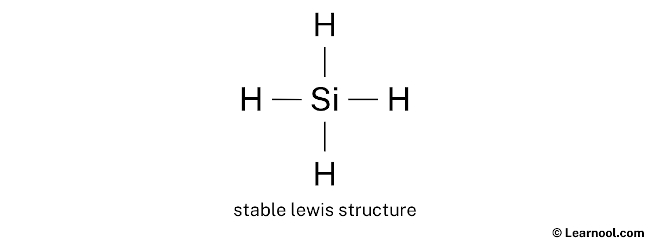
#2 Mark Lone Pairs
Hither, we accept a total of iv electron pairs. And four Si — H bonds are already marked. So we exercise non have to mark any electron pair equally a lone pair on the sketch.
In the higher up structure, you lot can run across that the key atom (silicon) forms an octet. And the outside atoms (hydrogens) also form a duet. Hence, the octet dominion and duet rule are satisfied.
Therefore, this structure is the stable lewis structure of SiH4.
Next: ClO4 – Lewis Structure
.
.
.
External Links:
- https://techiescientist.com/sih4-lewis-structure/
- https://www.thegeoexchange.org/chemistry/bonding/Lewis-Structures/SiH4-lewis-structure.html
- https://lambdageeks.com/sih4-lewis-construction/
Sih4 Lewis Structure Molecular Geometry,
Source: https://learnool.com/sih4-lewis-structure/
Posted by: schendelarting1987.blogspot.com


0 Response to "Sih4 Lewis Structure Molecular Geometry"
Post a Comment